In a historic move, the U.S. Food and Drug Administration has officially approved the use of pegcetacoplan, a new treatment for macular degeneration and geographic atrophy and the first treatment of its kind to slow the progression of late-stage retinal atrophy.
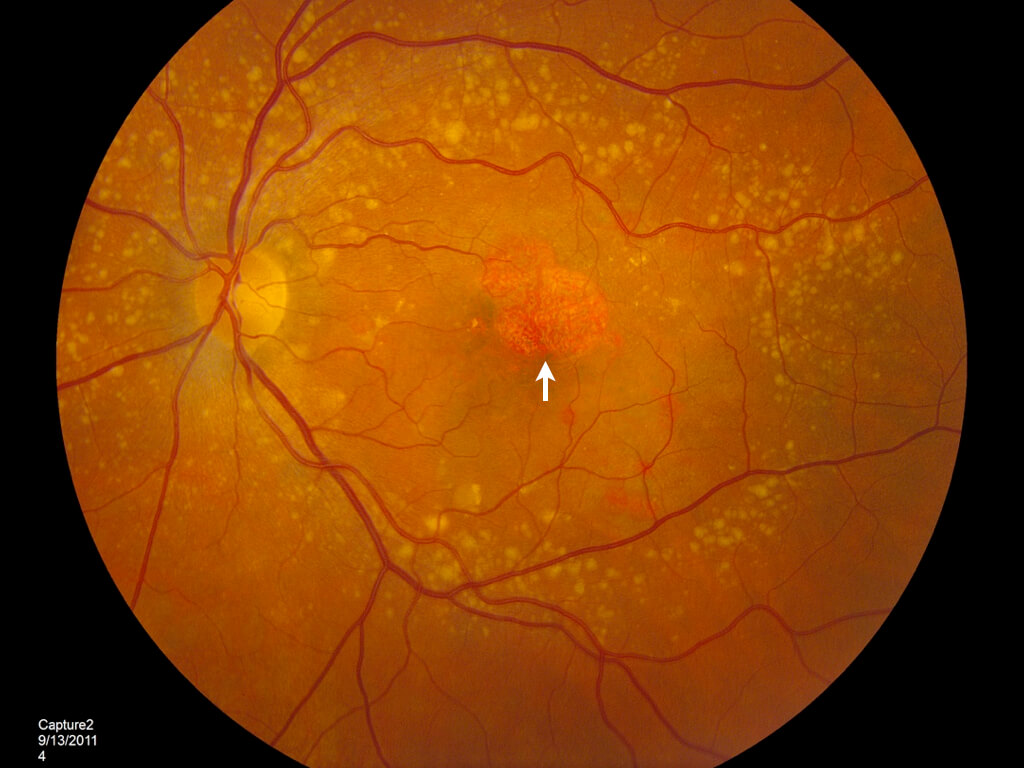
Age-related macular degeneration (AMD) is one of the most common eye conditions for older adults. At late stages, it develops into geographic atrophy (GA), a chronic and progressive degeneration of retinal cells. Both AMD and GA directly impact the macula, which is the part of the eye responsible for central vision. This brings with it the potential for permanent partial or full vision loss – an unfortunate reality for all too many AMD and GA sufferers.
Effective GA and AMD treatment options have long been lacking, but a solution is finally here. Pegcetacoplan is the first geographic atrophy treatment on the market, giving new hope to individuals dealing with late-stage AMD.
We’re proud to say that the physicians at Mid Atlantic Retina, as part of the world-renowned Wills Eye Hospital, were involved in the clinical trials leading to the approval of this new drug and treatment. Here’s what to know about it, including just how exciting this advancement is in the treatment of AMD.
Can dry macular degeneration be treated? For most of our medical history, the answer has been “no,” at least not in a meaningful way that can stop end-stage progression of the disease in its tracks.
This has all changed with the FDA approval of pegcetacoplan, an injectable retinal atrophy treatment and the very first of end-stage AMD treatment options.
“The FDA approval of a therapy to reduce the rate of growth of geographic atrophy is a landmark event,” said Carl Regillo, MD, director of retina services at Wills Eye Hospital, in an article for the Ophthalmology Times. “The benefit of a course of pegcetacoplan is a relatively small but significant step forward in managing an otherwise relentlessly progressive, visually disabling, end-stage form of dry AMD that was previously untreatable,” he added.
Pegcetacoplan works by regulating the excessive activation of the immune system’s complement cascade – the part of the immune system responsible for clearing microbes and damaged cells from the body, among other things. When out of balance, the complement cascade can lead to both the onset of disease and the progression of existing diseases, including the progression of AMD to GA.
While not entirely curative as a wet or dry AMD treatment, pegcetacoplan can be instrumental in halting the progression of the illness and preserving retinal cells. And in doing so, it can help individuals maintain their vision even in light of a progressive AMD diagnosis.
Approval of intravitreal pegcetacoplan was based on the results of three phases of clinical trials completed over a 24-month period.
In phase 3 of the clinical trial, the efficacy, safety, and tolerability of pegcetacoplan was compared against placebo injections across a broad range of GA patients. These randomized, double-masked studies were performed at various locations, including Mid Atlantic Retina, and further cemented the ability of pegcetacoplan to serve as an effective geographic atrophy treatment.
Pegcetacoplan was well tolerated in phase 3 studies and proved both its safety and efficacy for patients receiving monthly or bi-monthly dosing. Compared to other AMD treatment options, which may relieve symptoms but fail to stall progression, pegcetacoplan was found to not only slow down the progression of AMD to GA but to increase its efficacy at doing so over time.
This new drug marks the first time that patients and specialists have had a truly effective retinal atrophy treatment – a momentous advancement and a turning point in our ability to treat patients with AMD.
We are proud to have participated in bringing this novel geographic atrophy treatment to market as part of our ongoing commitment to the treatment of macular degeneration. As a recognized Center for Excellence, our team at Mid Atlantic Retina works hard to ensure that our patients always have access to cutting-edge treatments, and especially those that can protect vision in the face of chronic degenerative illness.
Please contact us today to learn more about pegcetacoplan or to schedule a consultation with a Mid Atlantic Retina specialist.